
The chemistry of free radicals is covered in The Basics. An organic synthesis which uses radical chemistry is in the Synthesis section, an the roles free radicals play in the environment and in our bodies is touched upon in the In Nature page. There is a short summary and a bibliography in the Conclusion.
Interesting Fact
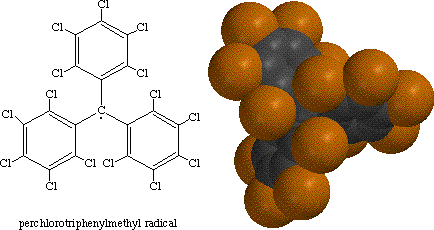
A free radical is normally very reactive, making them very difficult to isolate. An exception to this rule is the perchlorotriphenylmethyl radical, displayed below using a stick diargam and a spacefilling model. The chlorine atoms of the perchloro radical shield the central carbon radical from any molecule or atom which could react with it. As a result, at standard conditions, the radical is inert. In fact is unreactive at temperatures up to 300 degrees Celsius (Ege, 896).
![[Middlebury College]](../../images/middbutton.gif)
![[Class Material]](../../images/classbutton.gif)
![[Student Projects]](../../images/studentbutton.gif)
![[Software]](../../images/softwarebutton.gif)
![[Chemistry & Biochemistry]](../../images/chembutton.gif)
![[Molecular Modeling]](../../images/molmodbutton.gif)
![[WWW Sites]](../../images/wwwbutton.gif)
![[Main Page]](../../images/mainbutton.gif)
email me at:
burkett@mail.middlebury.edu