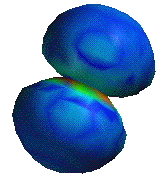
A chlorine atom, showing regions of spin density
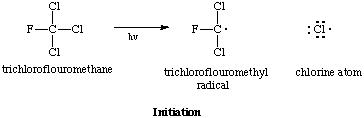
The ozone layer is located in stratoshpere. What makes chloroflourocarbons (CFC's) so dangerous is that they are stable enough to diffuse up to the stratosphere where they are abstrtactedby UV radiation. A typical CFC, trichloroflouromethane, cleaves to form two radicals: a highly reactive chlorine atom and a dichloromethyl radical. This is the initiation step in the ozone degradation chain reaction.
The chlorine atom that is created reacts with ozone to create chloride monoxide radical. Note that because two chlorine monoxide molecules are needed the initiation reaction must occur twice (see the following diagram). Two chlorine monoxide radicals dimerize and UV radiation breaks off one of the chlorine atoms. This causes the other chlorine atom to break lose, releasing molecular oxygen in the process. The two chlorine atoms then break down two more molecules of ozone.
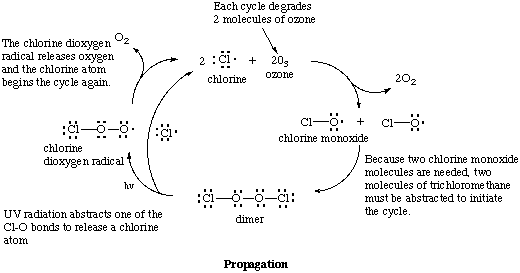
It is important to notice that no new chlorine atoms need to enter the cycle to keep it going; low numbers of chlorine atoms can catalyze the breakdown of many molecules of ozone. To stop the cycle two chlorine radicals have react with each other in a termination step.

Oxygen is what is known as a diradical; one of its primary resonance structures contains two unpaired electrons:
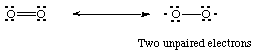
The fact that a molecule as prevelant as oxygen contains radical properties has many implications in the world around us. Molecules that are have the ability to stabilize unpaired electrons are vulnerable to the radical properties of oxygen. An example of such a compound is linoleic acid, shown below. Linoleic acid is a fatty acid very common in plant and animal cell membranes. Linoleic acid has a carbon atom which is allylic to two double bonds, meaning that an unpaired electron could be shared between three carbons, creating a fairly stable intermediate. The spin density of the linoleic acid molecule is painted on the model below. You can see how the unpaired electron is shared over three carbons and some hydrogen molecules.
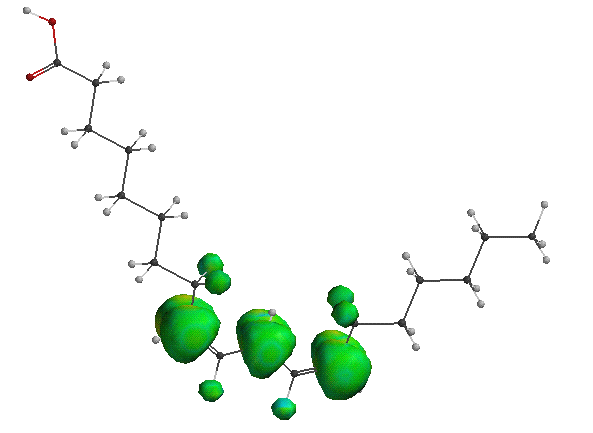
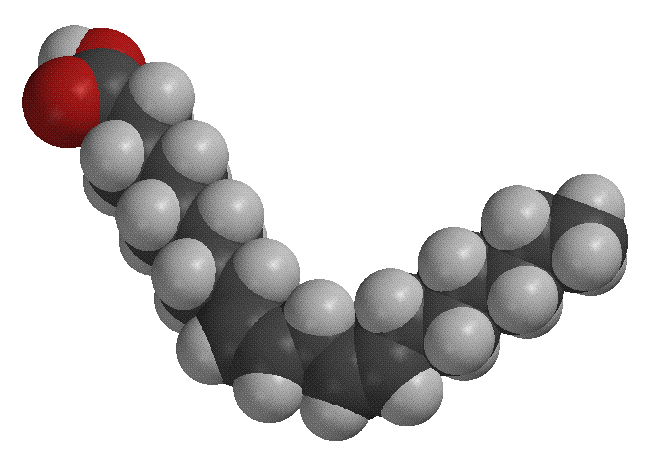
It is suspected that some type of peroxy radical attacks a hydrogen of the allylic carbon, creating the radical interemediate. The oxygen radical then attacks this radical, forming the molecule shown below on the right, which has a two conjugated double bonds and an oxygen molecule attached to it. (The figure on the left is another model of linoleic acid).
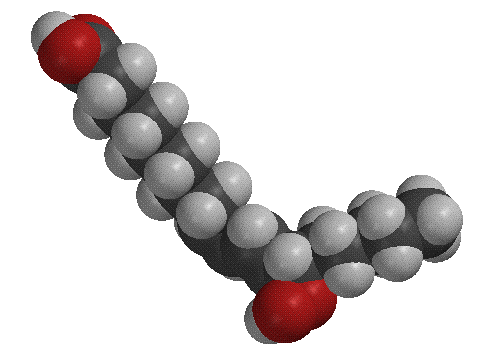
The breakdown of linoleic acid and other similiar polyunsaturated fatty acids is one of the major causes of food spoilage. When this oxidation occurs in a cell membrane you can imagine the disruption the polar tail of the product would have on the hydrophobic inner layer of the membrane.
Free radicals in the human body attack proteins, DNA and RNA, and fatty acids. There is definite corrletation between increased age and increased damage to these macromolecules (Delaasuncion 1996). It has not been proven for certain that this damage is the cause of aging, but there is a large body of evidence which supports this theory. One day some of that evidence will be explained on this page, but not as of yet.