A free radical is an atom or molecule which has an unpaired electron in its valence shell. Below are three different models of a radical of tert -butane in which a hydrogen atom has been abstracted from the tertiary carbon. On the left is a stick diagram, showing the bonding between atoms. In the middle is similiar model made three dimensional with Spartan. The model on the right is also made with Spartan. It depicts the highest occupied molecular orbital, or the homo, of the tert -butyl radical. The homo is the orbital which contains the highest energy electrons.
![]()
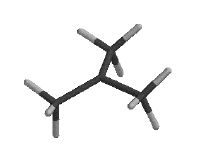
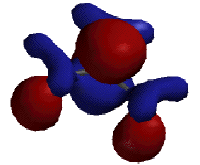
It is important to note that a free radical does not have to be, and normally isn't, charged; the tertiary carbon of tert -butyl radical does not have a filled valence shell, but the atom has the four electrons needed to make it neutral.
There are a number of ways radicals can be formed. A radical can result form the homolytic cleavage of a bond in a molecule. In bond which splits homolyticaly, one electon goes to each of the bonded atoms, creating two free radicals.
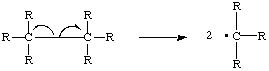
The 'fish hook' arrows represent the movement of a single electron. .The energy needed to break the bond can come from a variety of sources, including UV radiation, gamma rays, and intense heat. New radicals are also formed from existing radicals. Because an existing radical normally produces a new radical when it reacts, reactions involving radical intermediates are often chain reactions. The formation of free radicals from from non- radicals is known as the initiation step of a chain reaction (Ege ).
When a free radical forms a new bond with an atom by breaking an old bond it only uses one of the electrons from the old bond, leaving a new atom with an unfilled valence. This is known as abstraction (Ege ). For example, in the reaction below, a chlorine atom abstracts the hydrogen of a tertiary carbon. The initiation step of this reaction was the homolytic cleavage of molecular chlorine.
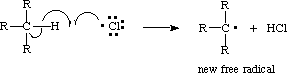
The reactants produced hydrochloric acid and a new tertiary carbon free radical. The new free radical will abstract a bond of another molecule, which, in this instance, might be molecular chlorine:
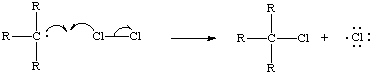
The products of this reaction are the reactants of the first reaction. The chlorine and tertiary carbon molecule can now become reactants and form another molecule of hydrochloric acid and another tertiary carbon radical. When the products of one reaction are the reactants for another it is known as a chain reaction. As long as two radicals don't react with each other this cycle will continue. It is called the propagation stage of a free radical chain reaction. When two radicals do react with each other the unpaired valence electrons form a bond and no further radicals are formed. It is the termination of a free radical chain reaction (Ege, ).
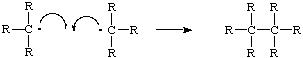
Propagation steps are much more common than termination steps due to the short life span of free radicals. In a an experimental reaction there are many molecules going through the reaciton at once and a termintaiton step would not stop the whole reaction (Ege, ).
Not all bonds have an equal chance of breaking to form a free radical, certain characteristics of a molecule effect how prone it is to free radical formation. Two factors that can make a molecule a good candidate are a high degree of substitution and resonance stabilization. Spartan can be used to show how they make a molecule a good free radical precursor.
Resonance
Spreading the burden of an unpaired electron over more than one atom makes a free radical more stable. The amount of stability resonance gives to a free radical can be seen by comparing the radicals of propene and propane.
![]()
The propene radical is the more stable of the two. It has the following resonance structures:
![]()
Propene radical spreads its unpaired electron between two carbons. This can be seen more clearly using the spin density surface in Spartan
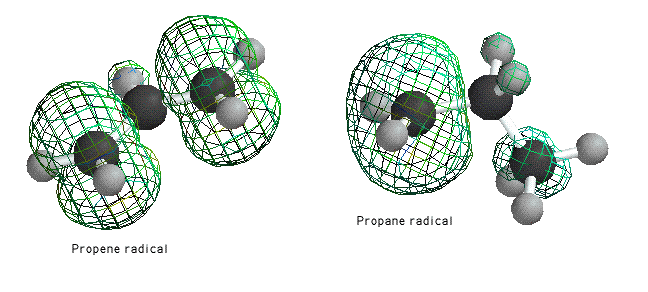
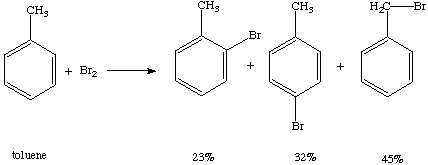
The spin density surface shows areas on a molecules which would be likely to contain the unpaired electron. As you can see, the propene radical has areas of spin density over both methyl groups, while the electron is isolated to one methyl group in the propane radical. Because of it has many resonance contributors, toluene can spread an unpaired electron over many atoms.
The delocalization of the radical electron is spread almost evenly between the carbon of the methyl group and the carbon atoms ortho and para to the methyl group. Because of the delocaliztion, if this compound were combined with molecular bromine a mixture of substitution products would be obtained (Ege, ):
Degree of Substitution
Spartan was used to calculate the heat of formation of three different free radical intermediates of 2-methyl butane: one in which the unpaired electron is on a tertiary carbon, one where the electron was on a secondary carbon and one where it is on a primary carbon.
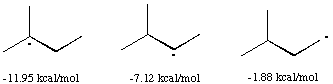
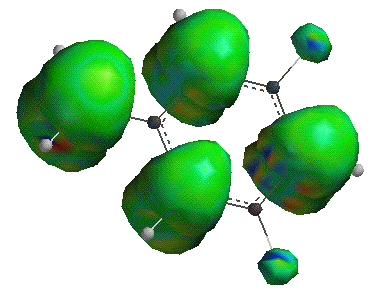
The interemediate with unpaired electron on the tertiary carbon has the lowest heat of formation, and is therefore the most stable. A high degree of substitution increases the stability of a carbon free radical intermediate for the same reason it increases the acidity of a hydrogen bonded to the same carbon; the other atoms have an electron withdrawing effect, lowering the energy of a free radical or of a carboanion intermediate. Spartan can be used to show this electron withdrawing effect using the spin density surface. Two molecules are shown, the one on the left is tert -butane with a hydrogen abstracted from the central carbon, and the one on the right a methyl radical.
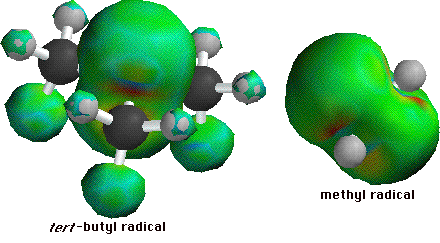
The green surfaces are the areas in which the unpaired electron would most likely be found. In the methyl radical, the unpaired electron is isolated to the carbon atom. In the tert -butyl radical the electron is shared between the central carbon and one hydrogen from each of the three methyl groupds. Notice the hydrogen atoms that share the electron are the ones whose sigma-bond line up with the central carbon's pi-orbital. One way to explain this is to discuss the stability that comes with higher degrees of substitution in terms of resonance. It is hypothesized that a resonance conformer exists in which the central carbon and one of the methyl carbons form a double bond, transfering the unpaired electron to the hydrogen atom which is bonded in the plane of the double bond. The surprising thing is that in this conformer the hydrogen atom is not bound to the carbon! Below are two views of the resonance structures. No attempt has been made to fill in the valence shells of the atoms.
![]()
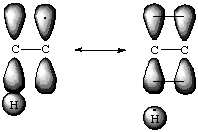
According to this theory, degree of substitution is just another form of resonance stabilization.