Home | Introduction | Oxygen Binding | F Helix Transition | Quarternary Shift | Synopsis | Bibliography

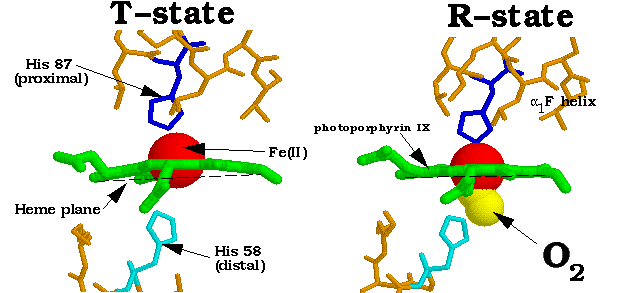
The Fe(II) atom at the center of the heme group is 5-coordinated by four nitrogen atoms from the surrounding porphyrin ring (green) and one nitrogen atom from the proximal histidine. Upon oxygenation of the subunit, the Fe(II) forms a sixth bond to the oxygen directly below the place of the heme group (opposite to the bond formed by the proximal histidine.) The binding of oxygen triggers a chain reaction of events that result in the entire Hb molecule undergoing a conformational shift from the low oxygen affinity T-state to the high affinity R-state. The shift begins when the bound oxygen changes the heme's electronic state, causing the Fe-porphyrin bonds to contract. This causes the heme to flatten out into a nearly planar molecule. When the heme flattens, the proximal histidine (bound to the Fe(II) atom) is pulled along, for appoximately 0.6 Å. This is only possible with an accompanying rearrangment of the attached F-helix; it is translated about 1 angstrom along the heme plane.