Home | Introduction | Oxygen Binding | F Helix Transition | Quarternary Shift | Synopsis | Bibliography

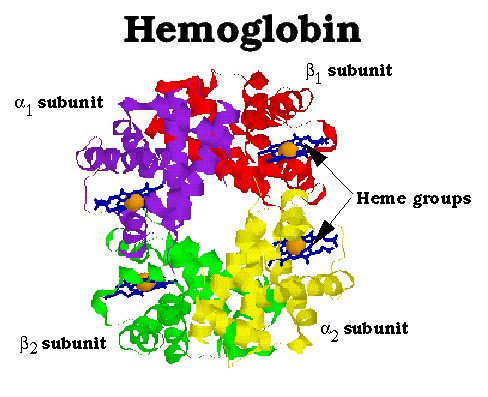
Hemoglobin (Hb) functions to transport oxygen in the blood of all warm-blooded animals. It is a multimeric protein consisting of four subunits, alpha1 , beta1, alpha2 and beta2. All four subunits resemble both themselves and myoglobin. The subunits are symetrically arranged; the above diagram depicts Hb viewed down its two-fold axis. Note the extensive contacts at between alpha and beta subunits, while there is little alpha-alpha and beta-beta contact (as they face each other across a 20 Å channel that runs through the center of the protein.) Also note the heme group located within a deep cleft in the side of each of the subunits. The heme groups are both the binding site for oxygen and the starting point for the mechanism of oxygen binding cooperativity.
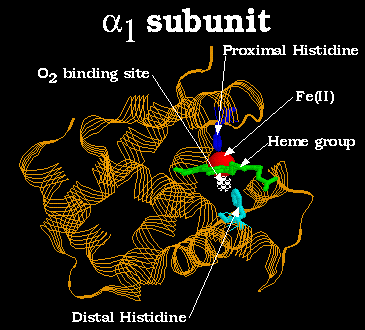
Above is a diagram of the alpha1 subunit of Hb. Oxygen binds to the Fe(II) atom, directly below the plane of the heme group and opposite the proximal histidine. Note how the top and bottom of the heme group are surrounded by the protein globule. This prevents two Hb heme groups from binding to the same oxygen molecule, which would allow one heme group to catalyze the autooxidation Fe(II) to Fe(III) in the other, resulting in irreversable oxygen binding.