Home | Introduction | Oxygen Binding | F Helix Transition | Quarternary Shift | Synopsis | Bibliography

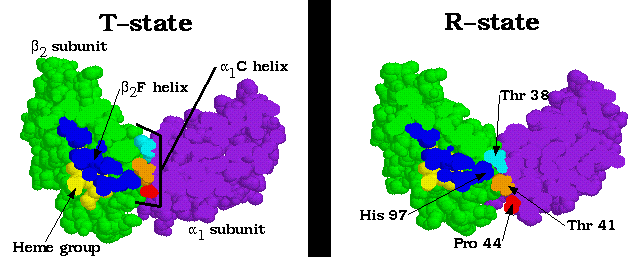
Recall from the introduction that there is exstensive contact between the alpha-beta interfaces in Hb. While the alpha1-beta1 and alpha2-beta2 interfaces are rigid, the alpha1-beta2 and alpha2-beta1 interfaces are somewhat flexible. This flexibility allows the translation of the F helix to result in a quarternary shift at the alpha1-beta2 and alpha2-beta1 interfaces. Shown above, the beta2 F helix terminus- His 97- moves down one turn of the alpha1 C helix, from in between the Pro 44 and Thr 41 residues to in between the Thr 41 and Thr 38 residues. Note that no intermediate position would be stable- the His 97 and Thr 41 residues would bump into each other. This accounts for the absence of any stable intermediate form between the T and R states. Accompanying the F helix translation is extensive breaking and reforming of salt bridges and hydrogen bonds, as illustrated below.
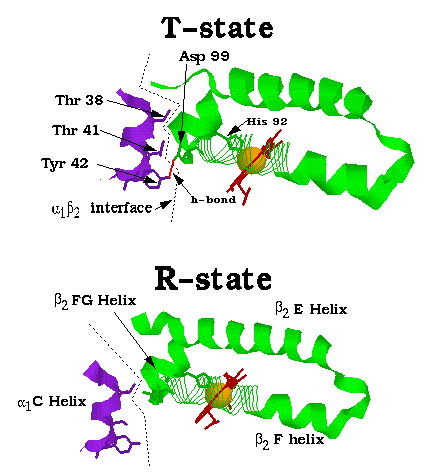
To the right is a closeup view of a portion of the alpha1-beta2 interface. It is possible to see the chain reaction of events that lead to the reorientation at the alpha1-beta2 (and alpha2-beta1) interfaces. Upon oxygen binding (not shown), the heme's doming is flattened, pulling the proximal his (here His 92) 0.6 Å towards the heme. This forces the attached F helix to undergo a 1 Å translation along the heme plane, pulling with it the attached FG helix. This movement results in the reoriention at the alpha1-beta2 interface, where the end of the F helix and the side of the FG helix are forced to break and reform many hydrogen bonds and salt bridges (The hydrogen bond between Tyr 42 and Asp 99 is one example).