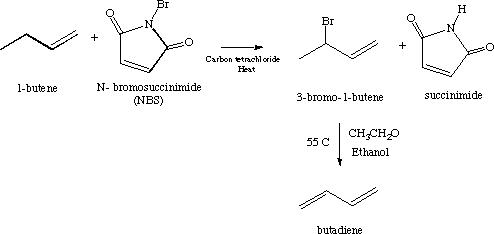
In the first reaction, the nitrogen-bromine bond breaks homolytically forming two free radicals:
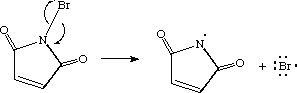
Bromine goes on to react with 1-butene to form a butene radical:

As shown in The Basics, the butene radical intermediate is stabilized by resonance:
![]()
Another molecule of NBS reacts with the hydrobromic acid created in the preceding step to form succinimide and molecular bromine.

The butene free radical abstracts the molecular bromine. This creates 3-bromo-butane and a bromine atom, one of the radicals present in the initial step. The bromine atom will propagate the chain reaction.

Because of the resonance stabilization of the allylic radical of butene, it is expected that 1-bromo-2-butene will also be produced.

Spartan can be used to estimate how important a product it will be.

The spin density shell shows that the two allylic carbons share the electron equally, meaning that 1-bromo-2-butene will probably be produced in high yield. This reaction is not pheasible experimentally, but it could be if the same molecule were produced whichever carbon bromine added to. This would be true in a cyclocarbon, such as cyclohexene:

.